Professional Factory for China Biobase Hospital Equipment Medicine Stability Test Chamber
The corporation upholds the philosophy of “Be No.1 in good quality, be rooted on credit history and trustworthiness for growth”, will continue to serve outdated and new shoppers from home and abroad whole-heatedly for Professional Factory for China Biobase Hospital Equipment Medicine Stability Test Chamber, Welcome to build the very well and extensive standing business enterprise interactions with our organization to make a wonderful long run together. customers’ pleasure is our eternal pursuit!
The corporation upholds the philosophy of “Be No.1 in good quality, be rooted on credit history and trustworthiness for growth”, will continue to serve outdated and new shoppers from home and abroad whole-heatedly for China Medicine Stability Test Chamber, Hospital Equipment, Our Company has specialist engineers and technical staff to answer your questions about maintenance problems, some common failure. Our product quality assurance, price concessions, any questions about the products, You should feel free to contact us.
The drug stability test chamber is based on a scientific method to create a long-term stable temperature and humidity environment for evaluating the expiration period of drugs to create evaluation conditions to meet the accelerated test, long-term test, high temperature or high temperature of the chemical drug stability test guidelines. The wet test is suitable for the stability inspection of drugs and new drug development in pharmaceutical companies.
|
Name |
Drug stability test chamber(Basic) |
Drug stability test chamber(Upgrade) |
||||
|
Model |
DRK-DTC-1 |
DRK-DTC-2 |
DRK-DTC-3 |
DRK-DTC-4 |
DRK-DTC-5 |
DRK-DTC-6 |
|
Temperature range |
0~65℃ |
|||||
|
Temperature fluctuation |
±0.2℃ |
|||||
|
Temperature uniformity |
±0.5℃ |
|||||
|
Humidity range |
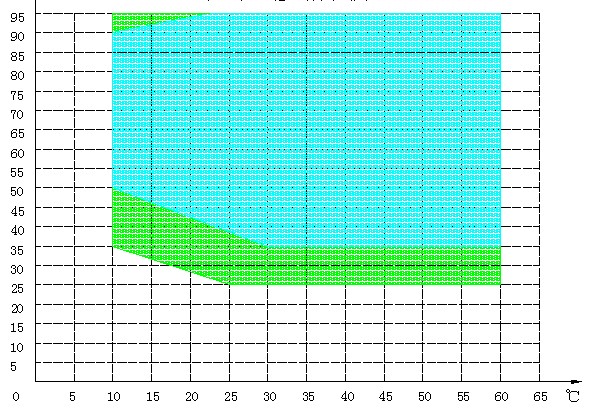
25~95%RH |
25~95%RH(20%~98%by customization) |
||||
|
Humidity deviation |
±3%RH |
|||||
|
Light intensity |
0~6000LX adjustable ≤±500LX,(Ten-level dimming, 600LX per level, precise control of intensity) Test distance 200mm |
0~6000LX adjustable ≤±500LX,(Stepless dimming) test distance 200mm |
||||
|
Timing range |
With 99 cycles of the program, each cycle is divided into 30 segments, each segment of 1~99 hoursof cyclic steps |
|||||
|
Light source board |
None |
None |
None |
None |
None |
None |
|
Temperature and humidity control method |
Balanced temperature and humidity control method |
|||||
|
Controller |
Large touch screen controller |
|||||
|
Ultraviolet energy lamp |
(Optional) Ultraviolet spectrum range 320~400nm |
Standard configuration) UV spectrum range 320~400nm, |
||||
|
Cooling system/method |
Fully automatic electronic expansion valve refrigeration control system/imported Danfoss compressor |
|||||
|
Temperature/humidity sensor |
Pt100 platinum resistance/imported German VAISALA humidity sensor |
|||||
|
Working temperature |
RT+5~30℃ |
|||||
|
Power supply |
AC 220V±10% 50HZ |
AC 380V±10% 50HZ |
AC 220V±10% 50HZ |
|||
|
Power |
1900W |
2200W |
3200W |
4500W |
1900W |
2200W |
|
Volume |
150L |
250L |
500L |
1000L |
150L |
250L |
|
WxDxH |
480*400*780 |
580*500*850 |
800*700*900 |
1050*590*1610 |
480*400*780 |
580*500*850 |
|
WxDxH |
670*775*1450 |
770*875*1550 |
1000*1100*1860 |
1410*890*1950 |
670*775*1450 |
770*875*1550 |
|
Loading tray(Standard) |
2pcs |
3pcs |
4pcs |
2pcs |
3pcs |
|
|
Embedded printer |
Standard configuration |
|||||
|
Safety equipment |
Compressor overheating protection, fan overheating protection, over temperature protection, compressor over pressure protection, overload protection, water shortage protection. |
|||||
|
Standard |
According to the 2015 edition of Pharmacopoeia drug stability test guidelines and GB/10586-2006 related manufacturing clauses | |||||
|
Numbering |
Content and description |
Standard |
|
URS1 |
Equipped with large touch control screen, touch screen≥7 inch. Real-time monitoring of operating conditions, can display current temperature (humidity), temperature (humidity) set value, date, time, temperature (humidity) curve and other working parameters. The operating parameters can be adjusted arbitrarily. |
Yes |
|
URS2 |
With data storage function, it can store 100,000 data. |
Yes |
|
URS3 |
With the user authority classification function, it can be divided into two user levels: technologist and operator.
Operator authority: view interface information, alarm and data curve functions. Technician authority: including operator authority, setting process parameters, area interface operation function, start and stop preset program, report query, operation record event query. Each account must log in with a password before performing operations within the scope of authority. |
Yes |
|
URS4 |
Equipped with an intelligent defrosting system, which does not affect equipment performance during defrosting. |
Yes |
|
URS5 |
The equipment is equipped with a micro printer (printing interval 0~9999 minutes). |
Yes |
|
URS6 |
The equipment has the main functions of heating, humidification, gating, lighting, sterilization, defrosting, and alarm. |
Yes |
|
URS7 |
Equipment operation mode is divided into: fixed value mode and program mode (program mode can be set for 30 segments and 99 cycles). |
Yes |
|
URS8 |
Equipment timing mode: running timing, constant temperature timing, constant humidity timing, constant temperature and humidity timing can be selected. |
Yes |
|
URS9 |
With alarm functions: temperature alarm, humidity alarm, water shortage alarm, door open alarm, etc. |
Yes |
|
URS10 |
Schedule switch machine function. |
Yes |
|
URS11 |
Power-off start function: No start: After power-off and restart, the system is in a stopped state.Hard start: After power off and restart, the system starts to run from the first segment of the first cycle, and the timing time is cleared.Soft start: After power off and restart, the system starts to run from the period of time when the power is off.The three startup modes can be switched freely, and the factory defaults to not start. |
Yes |
|
URS12 |
Standard USB interface, data can be exported instantly |
Yes |
The drug stability test chamber is based on a scientific method to create a long-term stable temperature and humidity environment for evaluating the expiration period of drugs to create evaluation conditions to meet the accelerated test, long-term test, high temperature or high temperature of the chemical drug stability test guidelines. The wet test is suitable for the stability inspection of drugs and new drug development in pharmaceutical companies.
The corporation upholds the philosophy of “Be No.1 in good quality, be rooted on credit history and trustworthiness for growth”, will continue to serve outdated and new shoppers from home and abroad whole-heatedly for Professional Factory for China Biobase Hospital Equipment Medicine Stability Test Chamber, Welcome to build the very well and extensive standing business enterprise interactions with our organization to make a wonderful long run together. customers’ pleasure is our eternal pursuit!
Professional Factory for China Medicine Stability Test Chamber, Hospital Equipment, Our Company has specialist engineers and technical staff to answer your questions about maintenance problems, some common failure. Our product quality assurance, price concessions, any questions about the products, You should feel free to contact us.
Products categories
-

Phone
-

E-mail
-

Whatsapp
-

Top












